Advanced Technology, High Responsibility
We manufacture sterile liquid, sterile lyophilized, and solid oral dosage forms in our two state-of-the-art production facilities. With a fully enclosed and isolator-based system infrastructure, we ensure the highest standards of safety and hygiene while protecting employee and environmental health. Our GMP-certified facilities continue to deliver reliable and innovative pharmaceutical solutions in full compliance with international quality standards.
Facility A (Sterile Liquid Products)
• Vial
• Pre-filled Syringe
Vial Capacity
18 Million Vials
Pre-filled Syringe Capacity
24 Million Syringes
In our vial filling line, we perform aseptic filling for volumes ranging from 2 ml to 250 ml, while in our pre-filled syringe (PFS) line, we handle volumes between 0.2 ml and 5 ml. We also carry out terminal sterilization processes, and meticulously execute post-filling operations such as optical inspection, labeling, blistering, and packaging.
Our production areas are designed as clean rooms in accordance with the A, B, C, and D cleanliness classes defined in the EU GMP guidelines. In this facility, we manufacture non-high potent products.
Facility B (High-Potency Products)
• Vial
• Pre-filled Syringe
• Lyophilized Powder
• Film-Coated Tablet
• Capsule
Vial Capacity
36 Million Vials
Pre-filled Syringe Capacity
24 Million Syringes
Tablet Production Capacity
240 Million Tablets
Capsule Production Capacity
35 Million Capsules
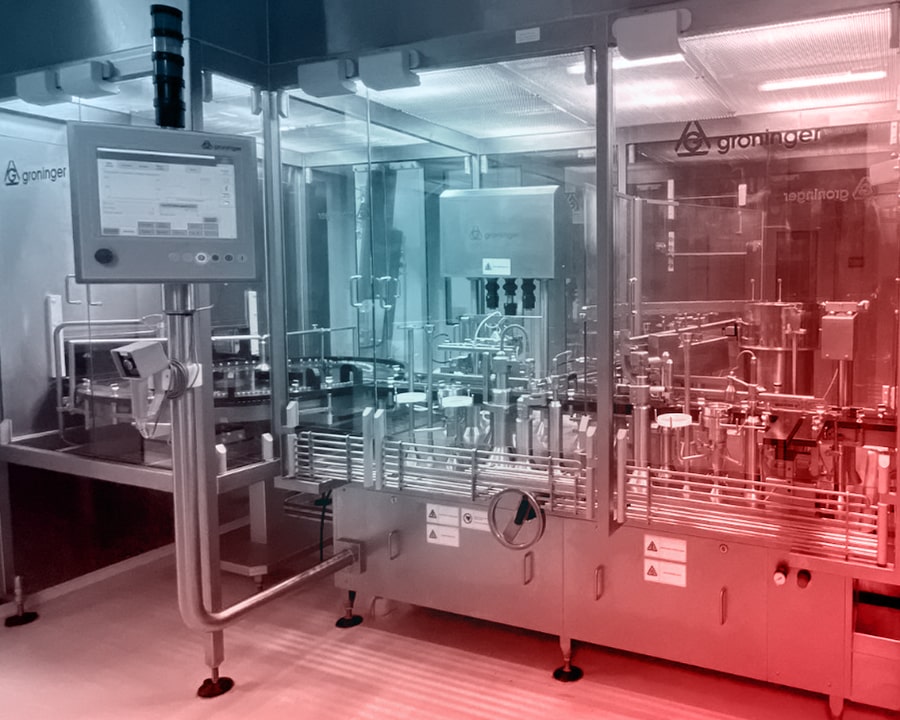
Sterile Liquid Manufacturing Department
In the sterile liquid production section, aseptic filling lines are capable of vial filling from 6 ml to 100 ml and pre-filled syringe (PFS) filling from 0.2 ml to 1 ml. Lyophilization and terminal sterilization processes are applied to ensure maximum production safety.
Post-filling operations, including optical inspection, labeling, blistering, and packaging, are meticulously performed, with the entire process carried out in fully enclosed isolator systems. In compliance with EU GMP standards, a specially designed cleanroom layout — featuring Grade A environments for internal areas and at least Grade D for surrounding areas — ensures the highest level of sterility.
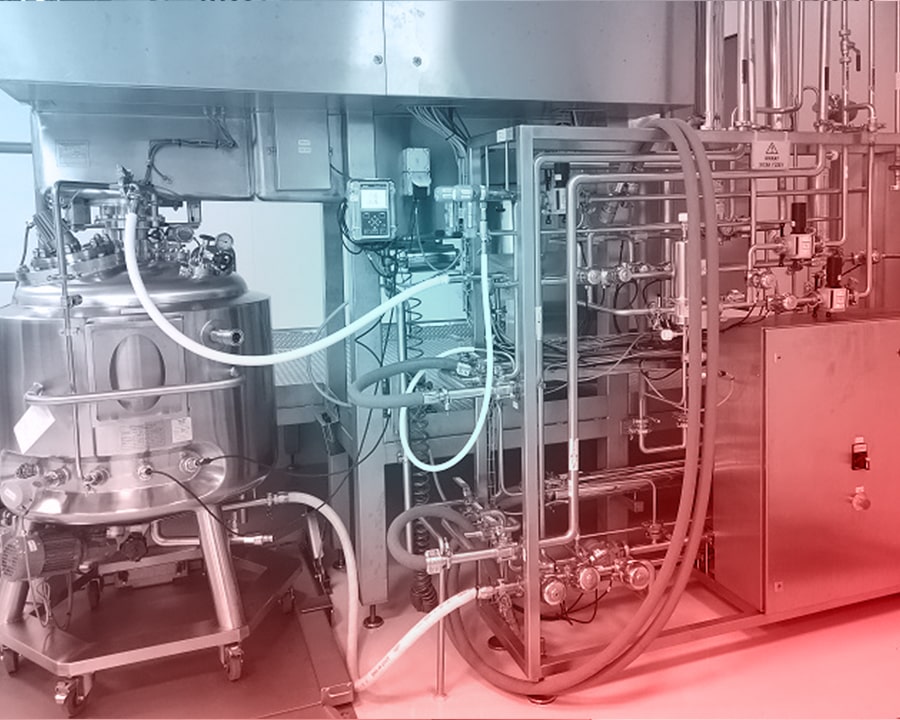
Isolator systems are also utilized during weighing and service preparation stages, aiming to protect employee health and prevent environmental contamination. In this section, where high-potent sterile liquid products are manufactured, sterile single-use hoses, connectors, filters, and bags are employed, each designed specifically for the process.
Solid Manufacturing Department
With our granulation, tablet, and capsule filling lines, we have the capacity to produce tablets and capsules in all sizes. Our tablet coating equipment enables us to perform additional processing, while we meticulously carry out post-production operations such as printing, blistering, and packaging.
All production processes are carried out in fully enclosed, isolator-based systems, adhering to the highest hygiene standards enabled by advanced technology. Operating in Class 100,000 clean areas, our facility ensures maximum safety by using isolator systems during weighing and all other production stages.
The infrastructure of this facility, where highly active solid products are manufactured, consists mainly of equipment from U.S. and European brands. The equipment used for highly active products is designed to maintain OEL 4/5 levels, representing the highest standards of operator protection and containment.